Most clients we work with are keen to communicate that their product is innovative. Of course they are. Innovation is important, right? Well, it is, but only if it benefits patients. In fact, in the context of reimbursement decisions for medicines, the definition of ‘rewardable’ innovation has evolved from being a new molecular entity to focus more on value or benefit.[1] And, sometimes, it’s the small, incremental developments that provide the most benefit. That being said, these incremental changes would not be possible without the more radical ‘game-changers’, which provide the starting point for further development. Therefore, a good treatment armamentarium relies on both radical and incremental innovation.
The treatment of diabetes is a perfect example of this. While there is still no cure for diabetes, the availability of increasingly effective medicines over the last century changed it from being considered a terminal diagnosis, particularly for those diagnosed in childhood, to a manageable, chronic disease.
Insulin: from radical innovation to standard of care
The key radical innovation that changed the course of disease for people with diabetes was the medical use of insulin in the 1920s. Before this, diabetes was managed with calorie restriction, which provided some benefit for those diagnosed in adulthood (those with what we now know as type 2 diabetes [T2D]) but did little for patients diagnosed in childhood (those with what we now know as type 1 diabetes [T1D]), who often died within days or weeks of diagnosis.[2] Insulin provided a treatment option that allowed those with access to treatment to live for many years after diagnosis.
Although an incredible breakthrough, initially, insulin was not the effective, well tolerated, and relatively convenient treatment that it is today. It was derived from animal sources and its use was complicated by issues of insulin resistance and supply.[3] Further innovation came with the introduction of human insulin and recombinant technology in the 1960s and 1970s, which helped to address these issues and make insulin more accessible and easier to use.[2],[3] Since these radical changes, a series of incremental innovations has resulted in availability of insulins that not only provide improved clinical outcomes, but also have varying durations of action to allow optimal glycaemic control while minimising the daily number of injections needed.[2] Advances in blood glucose monitoring and in the delivery of insulin through insulin pens and, more recently, pumps have also helped to make diabetes management easier for patients. Despite the advances in insulin therapy, a treatment burden remains, which can deter people from initiating and adhering to it.[4]
It’s not all about insulin
Innovation in the treatment of diabetes has not been limited to insulin. For those with T2D (approximately 90% of people with diabetes[5]), several additional treatment options are available, which may delay the need for insulin. For example, among other treatments, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) can be used for the treatment of T2D prior to initiation of insulin and can also be used in combination with insulin to improve disease control later in the treatment pathway.[6]
GLP-1 RAs provide another example of the benefits that incremental innovation can bring to patients. In addition to differences in efficacy and cardiovascular benefit seen within the GLP-1 RA class, incremental innovation within the class has resulted in substantial reductions in the burden of treatment on patients. The first GLP-1 RA used for diabetes (short-acting exenatide) became available in 2005 (US)/2006 (Europe) and required twice-daily injection. Since then, two GLP‑1 RAs requiring only once-daily injection have become available, followed by three which require only once-weekly injection (Figure 1).[7] Most recently, the first orally administered GLP-1 RA has become available, meaning that willingness and ability to self-inject is no longer a pre-requisite for GLP-1 RA therapy.[7],[8] These advances have the potential to improve patient uptake of and adherence to effective treatments, thereby improving outcomes.
Figure 1: Timeline of GLP-1 RA development[9],[10]
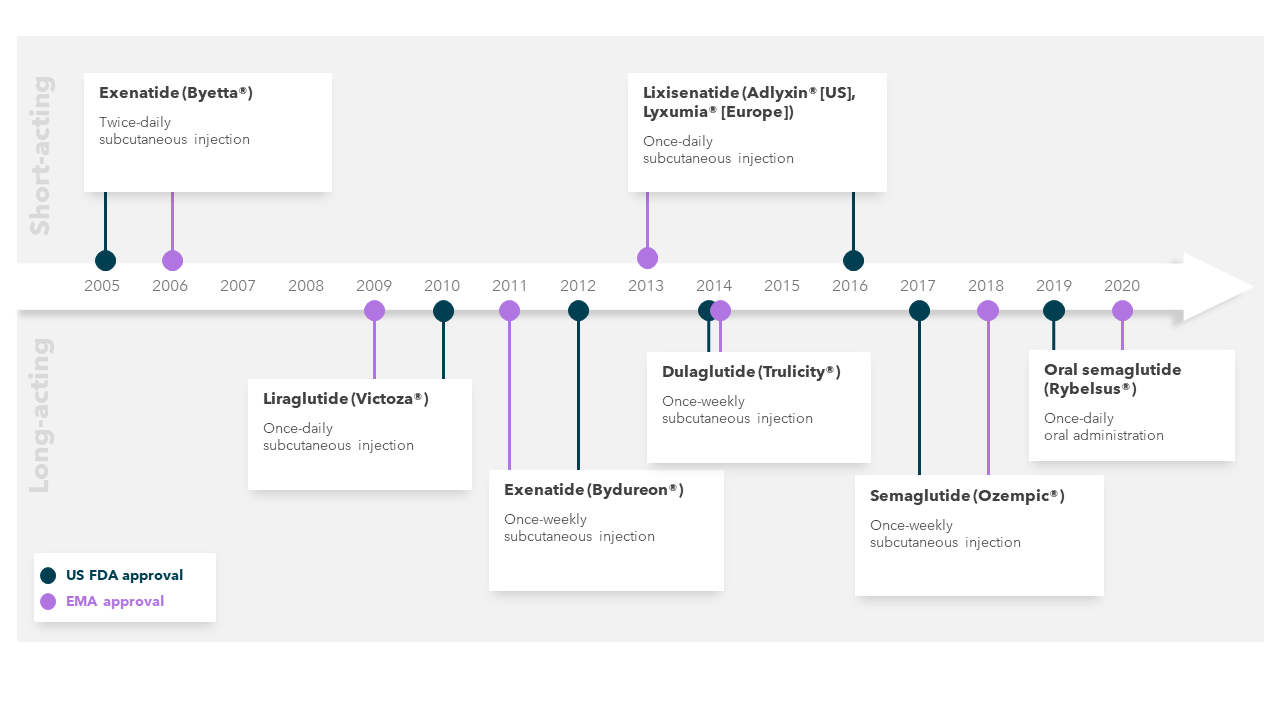
Abbreviations: EMA, European Medicines Agency; US FDA, United States Food and Drug Administration.
What’s next in diabetes?
Gene and stem cell approaches may eventually offer a cure for diabetes.[2] In the meantime, incremental innovation continues to provide treatment advances. For example, alternative formulations of insulin are expected to become available, including those requiring only once-weekly administration[11] and, potentially, even oral administration.[2] In addition, building on the success of the GLP-1 RA class, a dual glucose-dependent insulinotropic polypeptide and GLP-1 RA has recently become available and may provide even greater disease control than GLP-1 RA alone.[12]
While the search for a cure continues, a combination of radical and incremental innovation continues to dramatically alter the outlook for people with diabetes, not only improving outcomes, but also reducing the burden of treatment. The radical steps in innovation are, of course, necessary, but the impact of building on these steps through incremental innovation should not be underestimated. Ultimately, when it comes to reimbursement for a medicine, it is not the level of innovation but the level of patient benefit that matters most.
At Clarivate, we recognise that demonstrating value to market access stakeholders isn’t always straightforward, particularly when a product is adding to an existing treatment class. Such value demonstration requires an integrated approach to evidence generation planning, and the development of value propositions that are tailored to specific patient populations and unmet needs in a changing treatment landscape.
The Clarivate Value Communication team, part of Evidence, Value and Access Consulting, specialises in the development of robust and compelling value communication materials, reimbursement dossiers, objection handlers and publications. We also have extensive experience in primary payer and KOL research, and in developing interactive client workshops and training. To learn more about our capabilities and how we can support you, please get in touch at https://clarivate.com/products/life-sciences-and-healthcare-consulting-services/evidence-value-access-consulting/.
This post was written by Sophie Doran, Senior Director, Head of Medical Writing, and Sophie Streeton, Principal Medical Writer.
References
[1]Hofmann et al. A review of current approaches to defining and valuing innovation in health technology assessment. Value in Health. 2021; 24(12):1773-1783.
[2]Buse et al. 100 years on: the impact of the discovery of insulin on clinical outcomes. BMJ Open Diabetes Research and Care. 2021; 9:e002373.
[3]Hirsch et al. The evolution of insulin and how it informs therapy and treatment choices. Endocrine Reviews. 2020; 41:733-755.
[4]Zhu and Harris. Therapeutic inertia in people with type 2 diabetes in primary care: a challenge that just won’t go away. Diabetes Spectrum. 2020; 33(1):44-49.
[5]International Federation of Diabetes. Diabetes Atlas, 10th 2021.
[6]American Diabetes Association. Standards of Medical Care in Diabetes. 2023.
[7]Trujillo et al. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Therapeutic Advances in Endocrinology and Metabolism. 2021; 12:1-15.
[8]Aroda et al. A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. Reviews in Endocrine and Metabolic Disorders. 2022; 23:979-994.
[9]Food and Drug Administration. Drug approvals and databases. Available at: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases [Accessed November 2023].
[10]European Medicines Agency. Medicines. Available at: https://www.ema.europa.eu/en/medicines [Accessed November 2023].
[11]Rosenstock et al. Weekly icodec vs daily glargine U100 for type 2 diabetes without previous insulin. New England Journal of Medicine. 2023; 389:297-308.
[12]Frias et al. Tirzepatide versus semaglutide once-weekly in patients with type 2 diabetes. New England Journal of Medicine. 2021; 385:503-515.





